Ваше местоположение:Home > Импорт наркотикам
Traditional Chinese Medicine (TCM) of this annex refers to the medical substance and its preparation used under the guidance of Chinese traditional medical theory.
Natural Drugs of this annex refers to the medical natural substance and its preparation used under the guidance of modern medical theory.
Part I Registration Categories and Notes
I Registration Categories
1. active ingredients and its preparation extracted from plant, animal and minerals, which have not been marketed in China.
2. newly found drug material and preparations;
3. new TCM substitute
4. new part of drug material to be used as drugs,
5. new active part of material to be used as drugs and its preparation, which are extracted from plant, animal and minerals and have not been marketed in China;
6. compound preparation of TCM and natural drugs, which have not yet marketed in China
7. preparations with change in route of administration of the TCM or natural drugs already marketed in China,
8. preparations with change in dosage form of the TCM or natural drugs already marketed in China,
9. generic drugs.
II Notes
Drug under category 1-6 refers to as new drugs, while procedure for new drugs is applicable for the drug under category 7 and 8.
1. “active ingredients and its preparation extracted from plant, animal and minerals, which have not been marketed in China” refer to the single component or its preparation, which are extracted from plant, animal and minerals and not yet collected into National Drug Standards, where the content of this single component should be more than 90% of the extraction.
2. “newly found drug material and preparations” refer to the drug material and preparations not yet collected into National Drug Standard or provincial, autonomous region and municipal drug formulary (collectively called statutory standards)
3. “new TCM substitute” refer to drug material used to substitute the toxic drug material of the formula in the National Drug Standard or the endangered drug material, which is not yet collected by statutory standards.
4. “new part of drug material to be used as drugs” refer to the new part of existing drugs of animals or plants, which is to be used as drug, while the existing drugs is already in the statutory standards.
5. “new active part of material to be used as drugs and its preparation, which are extracted from plant, animal and minerals and have not been marketed in China” refer to active parts of similar or multiple component and its preparation, which are extracted from plant, animal and minerals, and not yet collected in National Drug Standards, where the active part should be more than 50% of the extraction.
6. “compound preparation of TCM and natural drugs, which have not yet marketed in China ” include
6.1 combined preparation of TCM
6.2 combined preparation of natural drugs,
6.3 combined preparation of TCM, natural drugs and chemical drugs
combined preparation of TCM should be formulated under traditional Chinese medical theory, including combined preparation of TCM from ancient classic formula, combined preparation with indication of ancient term, or combined preparation with combined term.
combined preparation of natural drugs should be formulated under modern medical theory, where indication should be in modern medical term.
combined preparation of TCM, natural drugs and chemical drugs include combined preparation of TCM and chemical drugs, combined preparation of natural drugs and chemical drugs, and combined preparation of TCM, natural drugs and chemical drugs
7. “preparations with change in route of administration of the TCM or natural drugs already marketed in China,” refers to the preparation with transfer between route of administration or absorption location.
8. “preparations with change in dosage form of the TCM or natural drugs already marketed in China” refers to the preparation of change in dosage form but with no change in route of administration.
9. “Generic drug” refers to the registration application of TCM or natural drug already approved to be marketed in China
Part II Application Information Items and notes
A Application Information Items
Summary information
1) Name of the drugs.
2) Certified Documents.
3) Objectives and basis for the application.
4) Summary and evaluation of main research results.
5) Sample draft of insert sheet, notes to the draft, and literature.
6) Sample design for packing and label.
Pharmaceutical Study Information
7) Summary of Pharmaceutical Study Information.
8) Source of the drug and determination.
9) Ecological environment, identity, description, cultivation and growing method, local processing and preparing method etc.
10) Draft of standard of drug material, and note of drafting, with provision of drug standard material and related information.
11) Sample of plant or mineral, sample of plant include flower, fruit, seeds etc
12) Research information of production process, verification information, and literature, source of excipients and quality standards.
13) Experiment data and literature of chemical content study
14) Experiment data and literature of quality study
15) Draft of the drug standards, with notes to the draft and verification with provision of drug standard material and related information.
16) Test report of sample.
17) Experiment data and literature of stability study.
18) Basis for selection and quality standards of immediate packing material and container
Pharmacology and Toxicology Study Information
19) Summary of the pharmacology and toxicology study information.
20) Experiment information and literature of pharmacodynamic.
21) Experiment information and literature of regular pharmacology study.
22) Experiment information and literature of acute toxicity.
23) Experiment information and literature of long term toxicity.
24) Special safety study and literature of hypersensitive (topical, systemic and photo-toxicity), hemolytic and topical irritative (blood vessel, skin, mucous membrane, and muscle) reaction related to topical and systemic use of the drugs.
25) Research information and literature of genotoxicity
26) Study and literature of reproductive toxicity.
27) Study and literature of carcinogenicity test.
28) Study and literature of animal pharmacokinetics.
Clinical Study Information
29) Summary of clinical study.
30) Clinical study plan and protocol.
31) Investigator’s Brochure. 32) Sample draft of Informed Consent Form, approval of the ethics committee.
33) Summary report of the clinical study.
II Notes
A, notes to application information items
Summary information
1. Information item 1, drug name includes,
i) Chinese name
ii) Phonetic name
iii) Nomenclature of the drug
2. Information item 2, Certified Documents, which include
i) Certified Documents of lawful registration of the Applicant, copies of Drug Manufacturing License, GMP Certificate. For the application of production of new drugs, copies of GMP Certificate for the workshop where the sample product of the drugs was manufactured should be provided.
ii) Certified Documents stating patent status and ownership of this entity and formula, production process of the drug, and letter of guarantee stating that no infringement upon the patent rights of others.
iii) Copies of official approvals of the research proposal of narcotics, psychotropic and medical-use toxic drugs.
iv) For the application of production of new drugs, copy of Approval of Clinical Study of New Drug.
ⅴ) Copies of the Drug Packing Material and Container Certificate or
Import Drug Packing Material and Container Certificate for the immediate packing material and container.
vi) Other certified documents.
If it is importation, the followings are also required
i) Certified Documents, notarized document for the free sale certificate (FSC) issued from the competent authorities of the local country or region where the manufacturer is located, the GMP Certificate of the manufacturer, and the certified Documents for exportation permit issued by competent authorities of the export country.
ii) When the registration of a foreign drug manufacturer is conducted by manufacturer’s office in China, copies of Registration Certificate Of Resident Office Of Foreign Enterprise should be provided.
When a foreign drug manufacturer authorizes domestic agent to conduct the registration, copies of the authorization document, notarized document and the Chinese translation, as well as the Business License of the domestic agent shall be provided.
iii) For safety experiment data, related GLP certificate should be provided, and GMP certificate should be provided for investigative drug for clinical trails.
3. Information Item 3, objectives and basis of the application, ancient and modern literature should be provided for TCM and natural drug, source of formula and basis for the application, current development of R&D in China and overseas, current clinical use and production summary, necessary analysis as for the innovation, feasibility, and rationale of dosage form, including the comparison with similar drug already with National Standard, should be provided for preparation of TCM and natural drug. For TCM, traditional medical theory and ancient drug book should also be provided.
4. Information Item 4, summary and evaluation of main research results, includes the summary of main research results by the Applicant, and a comprehensive analysis of safety, efficacy, and quality controllability of the drugs of the application.
5. Information Item 5, draft of insert sheet, notes to the draft and latest literature, includes the sample of draft of packaging insert sheet drafted in accordance with the relevant regulations, notes on how each items of the insert sheet were drafted, latest relevant literature etc.
Summary of Pharmaceutical Study
6. Information Item 16, self test report: refer to the self test repot of sample, report for at least one batch should be provided when applying for clinical trial, upon completion of clinical trial, self test report of 3 batch of project should be provided when submitting the dossier.
Summary of pharmacology and toxicology Study
7. Information Item 24, experiments information and literature related to topical and systemic use of the drugs: such as hypersensitive (topical, systemic and photo-toxicity), hemolytic and topical irritative (blood vessel, skin, mucous membrane, and muscle) reaction, Experiments information of safety of preparation should be provided according to the details of the route of administration and preparation. When there is a tendency of drug dependence, experiment data related to drug dependence should be provided.
8. information item 25: Experiments information and literature related to genotoxicity: if the formula include drug material not yet collected in statutory drug standards, or from the active part of drug material not yet collected in statutory drug standards, or new drug to be used for the child bearing patient group where it act on reproductive system (such as contraceptives, sexual hormones, drugs for sexual function disorder, drugs for maturation promoting of sperm, tocolytic drugs or drugs with cytotoxicity), genotoxicity test data should be provided.
9. information item 26, Experiments information and literature related to reproductive toxicity: for new drug to be used for the child bearing patient group where it may act on reproductive system (such as contraceptives, sexual hormones, drugs for sexual function disorder, drugs for maturation promoting of sperm, tocolytic drugs or drugs with cytotoxicity), reproductive toxicity test data should be provided according to the specific situation.
10. information item 27, Experiments information and literature related to carcinogenicity: during long term toxicity test of a new drug if cytotoxic effects were shown or extraordinary activation on the growth of cells in certain visceral organs and tissues were caused, or there is a positive test result during mutagenicity test, then experiments information and literature related to carcinogenicity must be provided.
B, requirement for application dossier
1. Information item 1-4 and 7-31 should usually be summated for application for clinical trails of new drug.
2. As to application for production upon completion of clinical trial, information item 1-33 as well as other changes and supplemental information should be submitted with detailed explanation of reason and basis.
3. Information item 2-8, 12 and 15-18 should be usually submitted for application of generic drugs(except for TCM or natural drug injection where clinical trail is needed).
4. All technical information and certified document from local authority used for importation application should be in Chinese attached with original document, where Chinese version of quality standard should be complied and submitted according to the format specified by Chinese National drug standards.
5. As for the complexity and diversity of TCM and natural drugs, when making the application, necessary research should be conducted according to the specific drug. If there is need for reduction or exemption of tests, there should be sufficient justified reasons.
6. Technical requirement of TCM and natural drug injection should be separately promulgated.
7. For the drug of category 1, active ingredients and its preparation extracted from plant, animal and minerals, which have not been marketed in China, if the active ingredients is related to the known carcinogen or the metabolite of the new drugs are similar to the known carcinogen, or if the expected treatment period is longer than 6 months, or used for treatment of chronic and recurrent disease, or intermittent use for a regular period of time, then experiments information and literature related to carcinogenicity must be provided. For the application for active ingredients and its preparation extracted from plant, animal and minerals, which have not been marketed in China, if there is similar drug or preparation made from active part extracted from single plant, animal and minerals, which have been marketed in China, then there should be pharmacodynamics comparison between the drug and the existing part to evidence the advantage of the new drug.
8. For the substitute of TCM material under registration category 3, in addition to the pre-clinical submission requirement of registration category 2, the experiment data of Pharmacodynamics comparison between this drug and the substituted drug should also be provided, while experiment and information of human tolerance test and clinical bio-equivalence test of the related preparation should be provided as well. If the substitute is a single component, experiment and literature of Pharmacokinetic test may be provided.
After the approval of the substitute of the TCM material, application for preparation of this substitute should follow the procedure of supplemental application, but must be strictly within the approved scope of substitution.
9. For those new active part of material to be used as drugs and its preparation, which are extracted from plant, animal and minerals and have not been marketed in China, which are under “registration category 5”, in addition to the required application information, the following information are also required to be submitted, i) research information or literature related to the screening of the active parts as required by information items 12, research information or literature related to major chemical content of the active parts as required by information items 13.
ii) If the active part is comprised of multiple components, each of the components should be assayed, where there should be lower limit of representative value for each component (upper limit should be added for the toxic component as well).
iii) When applying for new active part of material to be used as drugs and its preparation, which are comprised of similar content and extracted from plant, animal and minerals and have not been marketed in China, if it is comprised of active ingredient extracted from plant, animal and minerals that already marketed in China, pharmacodynamic comparison and other comparison should be conducted with this active ingredient to evidence the advantage and merit.
10. The required information for those under “registration category 6” that TCM, natural drugs and its combined preparation not yet marketed in China are as follows:
i) Combined preparation of TCM, some experiment data may be exempted according to the source of formula, indication and preparation process.
ii) Experiment and literature of efficacy and interaction of multiple components should be provided for combined preparation of natural drug.
iii) If the formula include drug not listed in the statutory drug standards, additional application information are required to be submitted according to the requirements of the corresponding registration category.
iv) There must be a statutory standards for any material of medical purpose that used in the combined preparation of TCM, natural drug and chemical drugs, where comparison experiment data and literature as for efficacy and toxicological interaction (improvement in efficacy, reduce toxicity or complimenting) between TCM, natural drug and chemical drugs, as well as interaction in bioavailability between TCM, natural drug and chemical drugs should be provided for application of clinical trials. When it is applied for production, information from clinical trial should evidence the necessity of the formula, where the experiment data of interaction in bioavailability between TCM, natural drug and chemical drugs should be provided. Chemical drug that used in formula (single or combined formula) must have been collected into the National Drug Standards.
11. For those under “registration category 8” that preparations with change in dosage form of the TCM or natural drugs already marketed in China, the advantage and merits of the new preparation should be explained. The indication of the new preparation should in principle be the same with that of the old preparation, if there is no way to verify by efficacy or clinical trail, the related information should be provided.
12. For those under “registration category 9”, generic drug should be consistent with the drug it copies from, and if necessary, the quality standards should be improved.
13. Clinical Study
i. Cases of patients for clinical trials should meet the statistical requirement and the minimal cases required.
ii. The minimal cases required (trial group) of clinical trials are as following, 20-30 for Phase I, 100 for Phase II, 300 for Phase III, 2000 for Phase IV,
iii. Phase Ⅳ clinical trail should be conducted for the new drug of registration category 1, 2, 4, 5 and 6, as well as those of category 7 and the drugs where there is significant change in process line or solvent.
iv. Bioequivalence trials should be normally 18-24 cases
v. Phase I clinical trial of the contraceptives should be conducted following this Regulation. In Phase II clinical trial, a randomized controlled clinical study should be conducted on at least 100 pairs of subjects for at least 6 menstruation cycles. In Phase III trial, an open trial on at least 1000 cases for 12 menstruation cycles should be accomplished. In Phase IV trial, variable factors of such kind of drugs should be carefully considered to finish the trial with adequate numbers of cases.
vi. For a new indication by the TCM substitute, preparation of the substitute that can sufficiently be representative of the indication of the substitute should be chosen from the drug standards and used as comparative drug for comparisons study, for each indication, more than 2 TCM preparations should be used for verification and clinical cases, for each preparation should not be less than 100 pairs of clinical trails.
vii. For those with change in dosage form, the clinical trails may be exempted or be conducted at no less than 100 pairs of cases according to the change in process and specific drugs.
viii. For generic drugs, the clinical trails shall be conducted at no less than 100 pairs of cases according to specific situation. ix. For imported TCM or natural drugs, application dossier should be provided according to the corresponding requirement of the registration category, where the research data and clinical trail data of the human pharmacokinetic study conducted in China should be provided, with no less than 100 pairs of clinical cases. For multiple indications, clinical cases for each major indication should not be less than 60 pairs.
Natural Drugs of this annex refers to the medical natural substance and its preparation used under the guidance of modern medical theory.
Part I Registration Categories and Notes
I Registration Categories
1. active ingredients and its preparation extracted from plant, animal and minerals, which have not been marketed in China.
2. newly found drug material and preparations;
3. new TCM substitute
4. new part of drug material to be used as drugs,
5. new active part of material to be used as drugs and its preparation, which are extracted from plant, animal and minerals and have not been marketed in China;
6. compound preparation of TCM and natural drugs, which have not yet marketed in China
7. preparations with change in route of administration of the TCM or natural drugs already marketed in China,
8. preparations with change in dosage form of the TCM or natural drugs already marketed in China,
9. generic drugs.
II Notes
Drug under category 1-6 refers to as new drugs, while procedure for new drugs is applicable for the drug under category 7 and 8.
1. “active ingredients and its preparation extracted from plant, animal and minerals, which have not been marketed in China” refer to the single component or its preparation, which are extracted from plant, animal and minerals and not yet collected into National Drug Standards, where the content of this single component should be more than 90% of the extraction.
2. “newly found drug material and preparations” refer to the drug material and preparations not yet collected into National Drug Standard or provincial, autonomous region and municipal drug formulary (collectively called statutory standards)
3. “new TCM substitute” refer to drug material used to substitute the toxic drug material of the formula in the National Drug Standard or the endangered drug material, which is not yet collected by statutory standards.
4. “new part of drug material to be used as drugs” refer to the new part of existing drugs of animals or plants, which is to be used as drug, while the existing drugs is already in the statutory standards.
5. “new active part of material to be used as drugs and its preparation, which are extracted from plant, animal and minerals and have not been marketed in China” refer to active parts of similar or multiple component and its preparation, which are extracted from plant, animal and minerals, and not yet collected in National Drug Standards, where the active part should be more than 50% of the extraction.
6. “compound preparation of TCM and natural drugs, which have not yet marketed in China ” include
6.1 combined preparation of TCM
6.2 combined preparation of natural drugs,
6.3 combined preparation of TCM, natural drugs and chemical drugs
combined preparation of TCM should be formulated under traditional Chinese medical theory, including combined preparation of TCM from ancient classic formula, combined preparation with indication of ancient term, or combined preparation with combined term.
combined preparation of natural drugs should be formulated under modern medical theory, where indication should be in modern medical term.
combined preparation of TCM, natural drugs and chemical drugs include combined preparation of TCM and chemical drugs, combined preparation of natural drugs and chemical drugs, and combined preparation of TCM, natural drugs and chemical drugs
7. “preparations with change in route of administration of the TCM or natural drugs already marketed in China,” refers to the preparation with transfer between route of administration or absorption location.
8. “preparations with change in dosage form of the TCM or natural drugs already marketed in China” refers to the preparation of change in dosage form but with no change in route of administration.
9. “Generic drug” refers to the registration application of TCM or natural drug already approved to be marketed in China
Part II Application Information Items and notes
A Application Information Items
Summary information
1) Name of the drugs.
2) Certified Documents.
3) Objectives and basis for the application.
4) Summary and evaluation of main research results.
5) Sample draft of insert sheet, notes to the draft, and literature.
6) Sample design for packing and label.
Pharmaceutical Study Information
7) Summary of Pharmaceutical Study Information.
8) Source of the drug and determination.
9) Ecological environment, identity, description, cultivation and growing method, local processing and preparing method etc.
10) Draft of standard of drug material, and note of drafting, with provision of drug standard material and related information.
11) Sample of plant or mineral, sample of plant include flower, fruit, seeds etc
12) Research information of production process, verification information, and literature, source of excipients and quality standards.
13) Experiment data and literature of chemical content study
14) Experiment data and literature of quality study
15) Draft of the drug standards, with notes to the draft and verification with provision of drug standard material and related information.
16) Test report of sample.
17) Experiment data and literature of stability study.
18) Basis for selection and quality standards of immediate packing material and container
Pharmacology and Toxicology Study Information
19) Summary of the pharmacology and toxicology study information.
20) Experiment information and literature of pharmacodynamic.
21) Experiment information and literature of regular pharmacology study.
22) Experiment information and literature of acute toxicity.
23) Experiment information and literature of long term toxicity.
24) Special safety study and literature of hypersensitive (topical, systemic and photo-toxicity), hemolytic and topical irritative (blood vessel, skin, mucous membrane, and muscle) reaction related to topical and systemic use of the drugs.
25) Research information and literature of genotoxicity
26) Study and literature of reproductive toxicity.
27) Study and literature of carcinogenicity test.
28) Study and literature of animal pharmacokinetics.
Clinical Study Information
29) Summary of clinical study.
30) Clinical study plan and protocol.
31) Investigator’s Brochure. 32) Sample draft of Informed Consent Form, approval of the ethics committee.
33) Summary report of the clinical study.
II Notes
A, notes to application information items
Summary information
1. Information item 1, drug name includes,
i) Chinese name
ii) Phonetic name
iii) Nomenclature of the drug
2. Information item 2, Certified Documents, which include
i) Certified Documents of lawful registration of the Applicant, copies of Drug Manufacturing License, GMP Certificate. For the application of production of new drugs, copies of GMP Certificate for the workshop where the sample product of the drugs was manufactured should be provided.
ii) Certified Documents stating patent status and ownership of this entity and formula, production process of the drug, and letter of guarantee stating that no infringement upon the patent rights of others.
iii) Copies of official approvals of the research proposal of narcotics, psychotropic and medical-use toxic drugs.
iv) For the application of production of new drugs, copy of Approval of Clinical Study of New Drug.
ⅴ) Copies of the Drug Packing Material and Container Certificate or
Import Drug Packing Material and Container Certificate for the immediate packing material and container.
vi) Other certified documents.
If it is importation, the followings are also required
i) Certified Documents, notarized document for the free sale certificate (FSC) issued from the competent authorities of the local country or region where the manufacturer is located, the GMP Certificate of the manufacturer, and the certified Documents for exportation permit issued by competent authorities of the export country.
ii) When the registration of a foreign drug manufacturer is conducted by manufacturer’s office in China, copies of Registration Certificate Of Resident Office Of Foreign Enterprise should be provided.
When a foreign drug manufacturer authorizes domestic agent to conduct the registration, copies of the authorization document, notarized document and the Chinese translation, as well as the Business License of the domestic agent shall be provided.
iii) For safety experiment data, related GLP certificate should be provided, and GMP certificate should be provided for investigative drug for clinical trails.
3. Information Item 3, objectives and basis of the application, ancient and modern literature should be provided for TCM and natural drug, source of formula and basis for the application, current development of R&D in China and overseas, current clinical use and production summary, necessary analysis as for the innovation, feasibility, and rationale of dosage form, including the comparison with similar drug already with National Standard, should be provided for preparation of TCM and natural drug. For TCM, traditional medical theory and ancient drug book should also be provided.
4. Information Item 4, summary and evaluation of main research results, includes the summary of main research results by the Applicant, and a comprehensive analysis of safety, efficacy, and quality controllability of the drugs of the application.
5. Information Item 5, draft of insert sheet, notes to the draft and latest literature, includes the sample of draft of packaging insert sheet drafted in accordance with the relevant regulations, notes on how each items of the insert sheet were drafted, latest relevant literature etc.
Summary of Pharmaceutical Study
6. Information Item 16, self test report: refer to the self test repot of sample, report for at least one batch should be provided when applying for clinical trial, upon completion of clinical trial, self test report of 3 batch of project should be provided when submitting the dossier.
Summary of pharmacology and toxicology Study
7. Information Item 24, experiments information and literature related to topical and systemic use of the drugs: such as hypersensitive (topical, systemic and photo-toxicity), hemolytic and topical irritative (blood vessel, skin, mucous membrane, and muscle) reaction, Experiments information of safety of preparation should be provided according to the details of the route of administration and preparation. When there is a tendency of drug dependence, experiment data related to drug dependence should be provided.
8. information item 25: Experiments information and literature related to genotoxicity: if the formula include drug material not yet collected in statutory drug standards, or from the active part of drug material not yet collected in statutory drug standards, or new drug to be used for the child bearing patient group where it act on reproductive system (such as contraceptives, sexual hormones, drugs for sexual function disorder, drugs for maturation promoting of sperm, tocolytic drugs or drugs with cytotoxicity), genotoxicity test data should be provided.
9. information item 26, Experiments information and literature related to reproductive toxicity: for new drug to be used for the child bearing patient group where it may act on reproductive system (such as contraceptives, sexual hormones, drugs for sexual function disorder, drugs for maturation promoting of sperm, tocolytic drugs or drugs with cytotoxicity), reproductive toxicity test data should be provided according to the specific situation.
10. information item 27, Experiments information and literature related to carcinogenicity: during long term toxicity test of a new drug if cytotoxic effects were shown or extraordinary activation on the growth of cells in certain visceral organs and tissues were caused, or there is a positive test result during mutagenicity test, then experiments information and literature related to carcinogenicity must be provided.
B, requirement for application dossier
1. Information item 1-4 and 7-31 should usually be summated for application for clinical trails of new drug.
2. As to application for production upon completion of clinical trial, information item 1-33 as well as other changes and supplemental information should be submitted with detailed explanation of reason and basis.
3. Information item 2-8, 12 and 15-18 should be usually submitted for application of generic drugs(except for TCM or natural drug injection where clinical trail is needed).
4. All technical information and certified document from local authority used for importation application should be in Chinese attached with original document, where Chinese version of quality standard should be complied and submitted according to the format specified by Chinese National drug standards.
5. As for the complexity and diversity of TCM and natural drugs, when making the application, necessary research should be conducted according to the specific drug. If there is need for reduction or exemption of tests, there should be sufficient justified reasons.
6. Technical requirement of TCM and natural drug injection should be separately promulgated.
7. For the drug of category 1, active ingredients and its preparation extracted from plant, animal and minerals, which have not been marketed in China, if the active ingredients is related to the known carcinogen or the metabolite of the new drugs are similar to the known carcinogen, or if the expected treatment period is longer than 6 months, or used for treatment of chronic and recurrent disease, or intermittent use for a regular period of time, then experiments information and literature related to carcinogenicity must be provided. For the application for active ingredients and its preparation extracted from plant, animal and minerals, which have not been marketed in China, if there is similar drug or preparation made from active part extracted from single plant, animal and minerals, which have been marketed in China, then there should be pharmacodynamics comparison between the drug and the existing part to evidence the advantage of the new drug.
8. For the substitute of TCM material under registration category 3, in addition to the pre-clinical submission requirement of registration category 2, the experiment data of Pharmacodynamics comparison between this drug and the substituted drug should also be provided, while experiment and information of human tolerance test and clinical bio-equivalence test of the related preparation should be provided as well. If the substitute is a single component, experiment and literature of Pharmacokinetic test may be provided.
After the approval of the substitute of the TCM material, application for preparation of this substitute should follow the procedure of supplemental application, but must be strictly within the approved scope of substitution.
9. For those new active part of material to be used as drugs and its preparation, which are extracted from plant, animal and minerals and have not been marketed in China, which are under “registration category 5”, in addition to the required application information, the following information are also required to be submitted, i) research information or literature related to the screening of the active parts as required by information items 12, research information or literature related to major chemical content of the active parts as required by information items 13.
ii) If the active part is comprised of multiple components, each of the components should be assayed, where there should be lower limit of representative value for each component (upper limit should be added for the toxic component as well).
iii) When applying for new active part of material to be used as drugs and its preparation, which are comprised of similar content and extracted from plant, animal and minerals and have not been marketed in China, if it is comprised of active ingredient extracted from plant, animal and minerals that already marketed in China, pharmacodynamic comparison and other comparison should be conducted with this active ingredient to evidence the advantage and merit.
10. The required information for those under “registration category 6” that TCM, natural drugs and its combined preparation not yet marketed in China are as follows:
i) Combined preparation of TCM, some experiment data may be exempted according to the source of formula, indication and preparation process.
ii) Experiment and literature of efficacy and interaction of multiple components should be provided for combined preparation of natural drug.
iii) If the formula include drug not listed in the statutory drug standards, additional application information are required to be submitted according to the requirements of the corresponding registration category.
iv) There must be a statutory standards for any material of medical purpose that used in the combined preparation of TCM, natural drug and chemical drugs, where comparison experiment data and literature as for efficacy and toxicological interaction (improvement in efficacy, reduce toxicity or complimenting) between TCM, natural drug and chemical drugs, as well as interaction in bioavailability between TCM, natural drug and chemical drugs should be provided for application of clinical trials. When it is applied for production, information from clinical trial should evidence the necessity of the formula, where the experiment data of interaction in bioavailability between TCM, natural drug and chemical drugs should be provided. Chemical drug that used in formula (single or combined formula) must have been collected into the National Drug Standards.
11. For those under “registration category 8” that preparations with change in dosage form of the TCM or natural drugs already marketed in China, the advantage and merits of the new preparation should be explained. The indication of the new preparation should in principle be the same with that of the old preparation, if there is no way to verify by efficacy or clinical trail, the related information should be provided.
12. For those under “registration category 9”, generic drug should be consistent with the drug it copies from, and if necessary, the quality standards should be improved.
13. Clinical Study
i. Cases of patients for clinical trials should meet the statistical requirement and the minimal cases required.
ii. The minimal cases required (trial group) of clinical trials are as following, 20-30 for Phase I, 100 for Phase II, 300 for Phase III, 2000 for Phase IV,
iii. Phase Ⅳ clinical trail should be conducted for the new drug of registration category 1, 2, 4, 5 and 6, as well as those of category 7 and the drugs where there is significant change in process line or solvent.
iv. Bioequivalence trials should be normally 18-24 cases
v. Phase I clinical trial of the contraceptives should be conducted following this Regulation. In Phase II clinical trial, a randomized controlled clinical study should be conducted on at least 100 pairs of subjects for at least 6 menstruation cycles. In Phase III trial, an open trial on at least 1000 cases for 12 menstruation cycles should be accomplished. In Phase IV trial, variable factors of such kind of drugs should be carefully considered to finish the trial with adequate numbers of cases.
vi. For a new indication by the TCM substitute, preparation of the substitute that can sufficiently be representative of the indication of the substitute should be chosen from the drug standards and used as comparative drug for comparisons study, for each indication, more than 2 TCM preparations should be used for verification and clinical cases, for each preparation should not be less than 100 pairs of clinical trails.
vii. For those with change in dosage form, the clinical trails may be exempted or be conducted at no less than 100 pairs of cases according to the change in process and specific drugs.
viii. For generic drugs, the clinical trails shall be conducted at no less than 100 pairs of cases according to specific situation. ix. For imported TCM or natural drugs, application dossier should be provided according to the corresponding requirement of the registration category, where the research data and clinical trail data of the human pharmacokinetic study conducted in China should be provided, with no less than 100 pairs of clinical cases. For multiple indications, clinical cases for each major indication should not be less than 60 pairs.
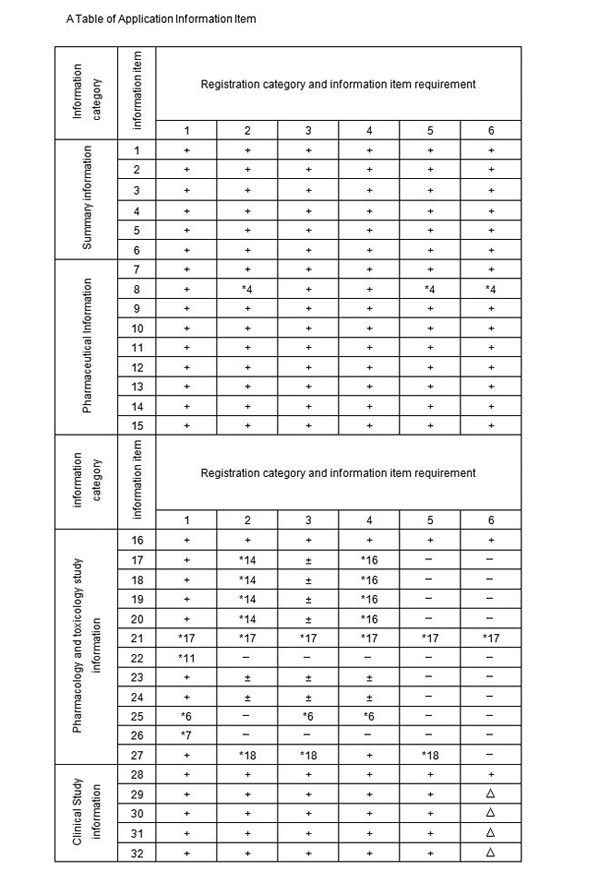
III Table of Application Information Item and notes
A Table of Application Information Item for TCM and natural drugs
Notes:
1. + Denote the information must be submitted,
2. − Denote the information may be exempted,
3. ± Denote literature can be used instead of test information or may be exempted by regulation,
4. ▲ Denote the information may not be provided for those of TCM and natural drug listed in statutory standards, and if not listed, data must be submitted.
5. * Denote the information shall be submitted according to the requirement